Weve got the study and writing resources you need for your assignmentsStart exploring. Solution for The most intense absorption band in the visible spectrum of MnH2O² is at 24900 cm and has a molar absorptivity of 0038 L mol cm.
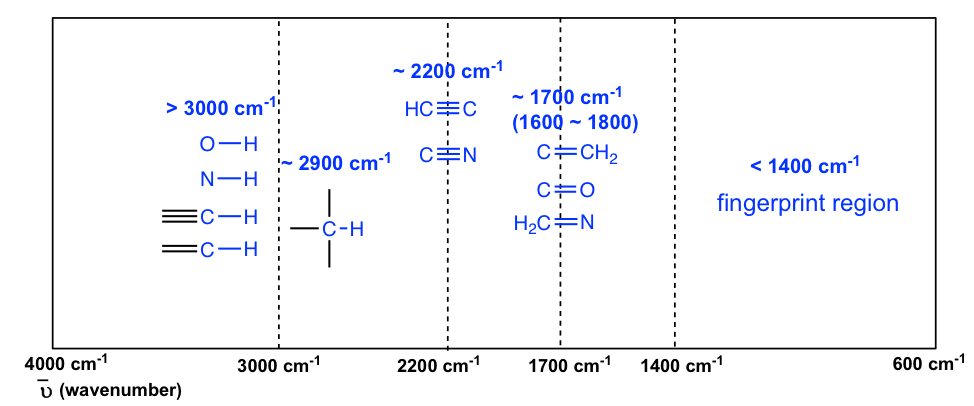
6 3 Ir Spectrum And Characteristic Absorption Bands Organic Chemistry I
Create a New Plyalist.

Intense absorption band. The CO stretch is much more intense than the CC stretch. The intense visible absorption band of native azurin and the corresponding transitions of the NiII and the CoII derivatives were analyzed. The fundamental measurement obtained in infrared spectroscopy is an infrared spectrum which is a plot of measured infrared intensity versus wavelength or frequency of light.
For example an aldehyde CO. The SchumannRunge continuum very strong between 135 and 176 nanometres. The Intensity of Absorption Bands.
The intensities of absorption band around 200 and 215 nm increase with increasing absorption dose in linear form while that around 275 nm is nearly constant. The most important factor that influences the intensity of an IR absorption band is the change in dipole moment that occurs during a vibration. Eor example benzene has several ultraviolet absorption bands due to 7t 71 transitions.
Enter Friends Emails Share Cancel. The CO bond is highly polar so its dipole. THE ABSORPTION BAND An absorption band is a range of wavelength frequencies or energies in the electromagnetic spectrum which are characteristic of a particular transition from initial to final state in a substance.
The intensity of an absorption band is always proportional to the following factors. School Western Oregon University. The probability involved is proportional to the population of.
Band intensity The absorptivities of fundamental bands in the condensed-phase spectra of most samples vary by well over an order of magnitude but the strongest band in the spectra of typical neat liquids or solids usually has an absorbance of between about 05 and 2 AU if the thickness of the sample is 10 pm. The absorption spectrum of the complex ion leftmathrmRhleftmathrmN Add To Playlist Add to Existing Playlist. The intense absorption band at 280 nm is due to the extraction of flavonols hydroxycinnamic acids flavanols and the UV absorption part of the anthocyanins.
The band observed around 320 nm is purely ascribed to the hydroxycinnamic acids. The bands were shown to be analogous ligand-to-metal charge transfer transitions and the optical electronegativity of the ligand involved is estimated to be 26. Butyraldehyde has an intense CO.
Finally the band observed at 520 nm is due to the anthocyanin extraction during fermentation. The position and intensity of two of these bands 2035 nm 8 7400 and 254 nm 8 204 are. Comparisons with small molecule systems.
An alkene CC stretch usually occurs near 1650 cm¹. Stretch usually occurs near 1730 cm¹. Course Title CHEMISTRY 332.
Source publication The best absorption lines for. The most intense absorption bands of CO CO2 and H2O in the spectral range of 1800-7000 cm -1. The intensity of a absorption band depends on the polarity of the bond the bond with higher polarity will show more intense absorption band.
Find an answer to your question Intense absorption band around 1700cm-1 indicates the presence of ----- group 1 point -OH COOH -CN -CO snmdarbar snmdarbar 2 weeks ago Science. The absorption bands in IR spectra have different intensity that can usually be referred to as strong s medium m weak w broad and sharp. Molecular population of the initial state as this is the initial state is the most influencing factor influencing the.
The absorptivity of strong bands in. Pages 24 This preview shows page 15 -. For example an aldehyde CO stretch usually occurs near 1730 cm¹.
Absorption at 1731 cm¹. Find the latest published documents for Intense Absorption Band Related hot topics top authors the most cited documents and related journals. Infrared spectroscopy is the study of the interaction of infrared light with matter.
The Hopfield bands very strong between about 67 and 100 nanometres in the ultraviolet named after John J. Download Table Maximums of the more intense induced absorption bands nm and the oscillator strengths of the transitions from publication. 1114015 Reg900048 Sesssion.
This is only a rule of thumb however. More intense the absorption band and the number of. The most intense absorption band in the visible spectrum of MnH2O62 is at 24900 cm-1 and has a molar absorptivity of 0038 L mol-1 cm-1.
What concentration of MnH2O62 would be necessary to give an absorbance of 010 in a cell of path length 100 cm. A diffuse system between 1019 and 130 nanometres. The absorption band of the carbonyl group of ketones is found in the region of two 1730 to 1700 cm COOH has two characteristic absorption.
The energy at which the absorption occurs as well as the intensity of the absorption is determined by the chemical environment of the absorbing moiety. The most important factor that influences the intensity of an IR absorption band is the change in dipole moment that occurs during a vibration. More intense the absorption band and the number of bonds responsible for the.
Theoretic and Experimental Study of Photoprocesses in. The results may indicate that these radicals and unsaturated bond contribute to the increase of electrical conductivity for the irradiated samples.
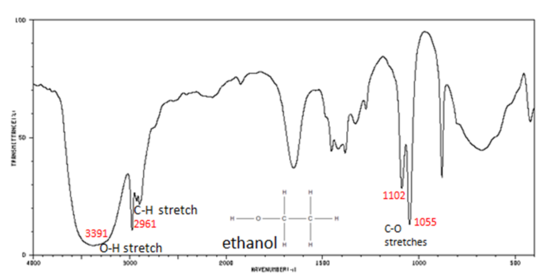
11 5 Infrared Spectra Of Some Common Functional Groups Chemistry Libretexts

A Location And Intensity Of Vibrational Rotational Absorption Bands Of Download Scientific Diagram
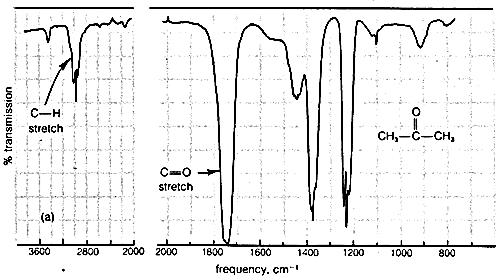
9 8 Infrared Rovibrational Spectroscopy Chemistry Libretexts

Atr Ir Spectrum Of Water On Znse Ire Download Scientific Diagram

A Absorption Spectra Of Tcp In Benzene For Different Concentration At Download Scientific Diagram
What Are The Factors That Influence The Intensity Of An Ir Absorption Band Socratic

Uv Vis Spectral Properties Of Individual Phenolic Compounds A Download Scientific Diagram
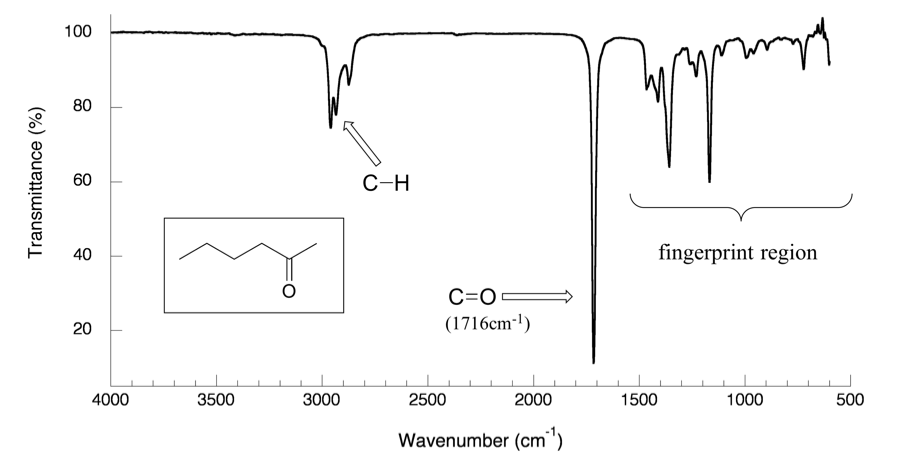
6 3 Ir Spectrum And Characteristic Absorption Bands Organic Chemistry I

Intensity Of Infrared Band An Overview Sciencedirect Topics

Absorption Maxima An Overview Sciencedirect Topics
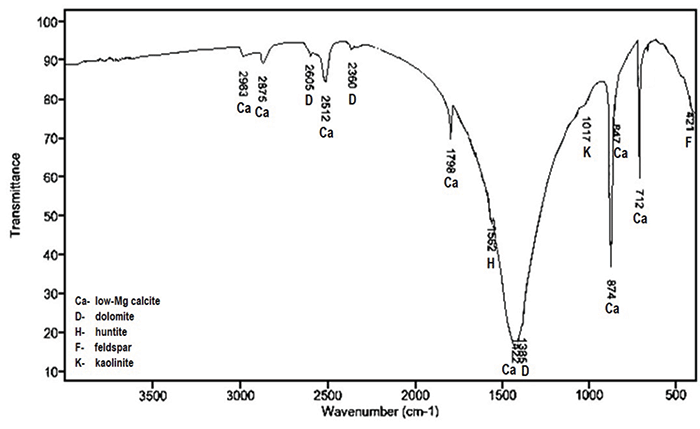
The Importance Of Fourier Transform Infrared Spectroscopy In The Identification Of Carbonate Phases Differentiated In Magnesium Content

Electronic Absorption Spectroscopy An Overview Sciencedirect Topics

Biopolymer Spectroscopy Ppt Video Online Download

Infrared Band An Overview Sciencedirect Topics
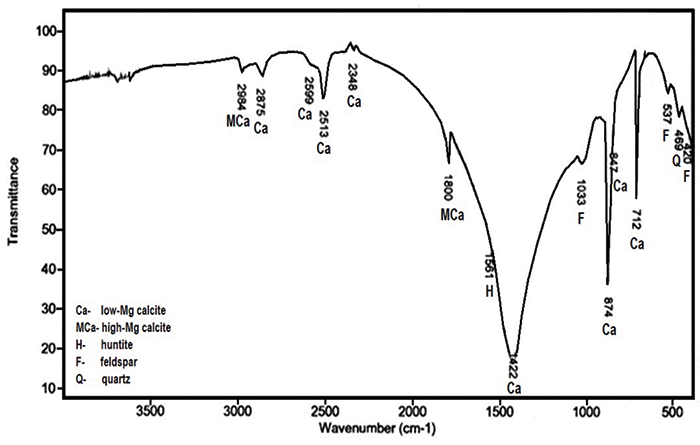
The Importance Of Fourier Transform Infrared Spectroscopy In The Identification Of Carbonate Phases Differentiated In Magnesium Content

Absorption Bands In The Near Infrared Download Scientific Diagram

Spectrum Of Absorption Of The Complexes Co H2o 6 2 And Co H2o 4 2 Download Scientific Diagram

The Most Intense Absorption Bands Of Co Co2 And H2o In The Spectral Download Scientific Diagram
